
Blood-based microRNA liver biomarkers – hepatomiR®
The hepatomiR® kit (CE-IVD) is intended for the quantification of hsa-miR-122-5p, hsa-miR-192-5p, and hsa-miR-151a-5p in human plasma samples for diagnostic use.
A software application included with the kit computes a liver function score (hepatomiR® P-Score) using a proprietary algorithm.
The hepatomiR® P-Score has been proven useful to assess the risk of post hepatectomy liver failure (PHLF) in patients with HCC, CCC, or metastatic colorectal cancer (Starlinger et al., HEPATOLOGY, Vol. 69, No. 6, 2019). This is critical to identify patients with a high risk of postoperative liver dysfunction and to plan appropriate risk reduction measures.
Other currently investigated intended uses of the product are the diagnosis acute drug-induced liver injury and the diagnosis and monitoring of metabolic associated liver disease (MAFLD).

assay characteristics
Quality: the only CE-certified in-vitro diagnostic RT-qPCR assay for robust quantification of three human microRNA liver biomarkers in plasma.
One-stop-shop: contains all necessary reagents and quality controls required to generate high-quality results. A software application allows instant transformation of raw data into easily interpretable figures and tables.
Broad applicability: analysis of miR-122-5p, miR-192-5p, and miR-151a-5p informs on liver function in the context of chronic or acute liver diseases: potential applications include the pre-operative assessment of liver function in HCC, CCC, and mCRC, the diagnosis of drug-induced liver injury (DILI), or the monitoring of non-alcoholic fatty liver disease (NAFLD).
Uniqueness: the only CE-certified test for the quantitative measurement of miR-122-5p, miR-192-5p, and miR-151a-5p in human plasma samples.
assay requirements
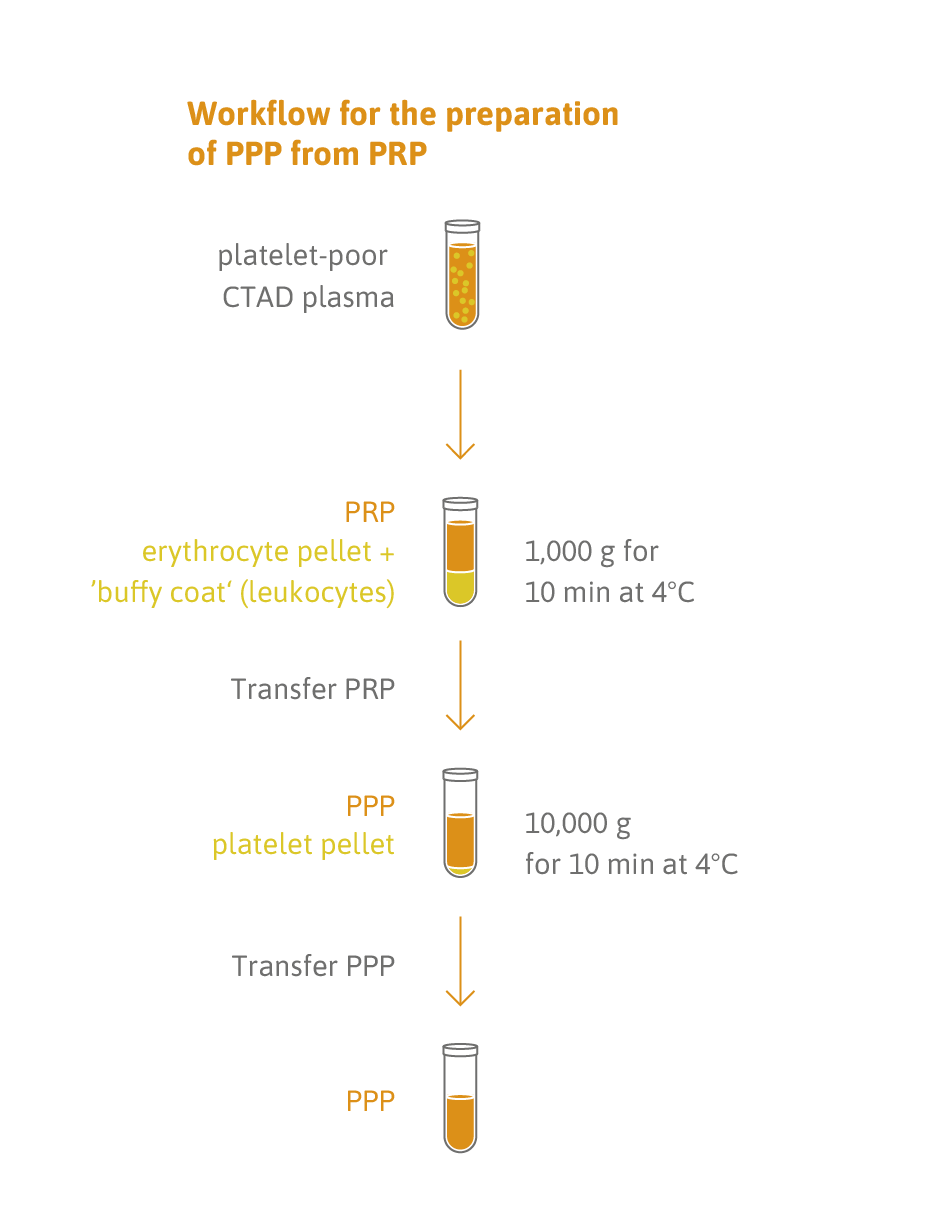
Sample type: 200 μl fresh or frozen platelet-free plasma.
Required equipment: qPCR instrument with 96-well or 384-well block. Roche, Biorad, and Quantstudio instruments are supported (see table below).
Not familiar with RT-qPCR?
Contact us to take advantage of our analytical services.
price list
| product number | product | size | PCR Cycler compatibility | price | product information |
|---|---|---|---|---|---|
| KT-031-HEP 96A | hepatomiR® 96-well |
48 samples 6 samples/96-well plate |
QuantStudio 5 / 5 Dx (96-well Standard Block) | price on request | instructions for use (IFU) download |
| KT-031-HEP 96D | hepatomiR® 96-well |
48 samples 6 samples/96-well plate |
Bio-Rad CFX96™ | price on request | instructions for use (IFU) download |
| KT-031-HEP 96F | hepatomiR® 96-well |
48 samples 6 samples/96-well plate |
Roche® LightCycler® 480 | price on request | instructions for use (IFU) download |
| KT-031-HEP 384G | hepatomiR® 384-well |
48 samples 24 samples/ 384-well plate |
Roche® LightCycler® 480 | price on request | instructions for use (IFU) download |
product description
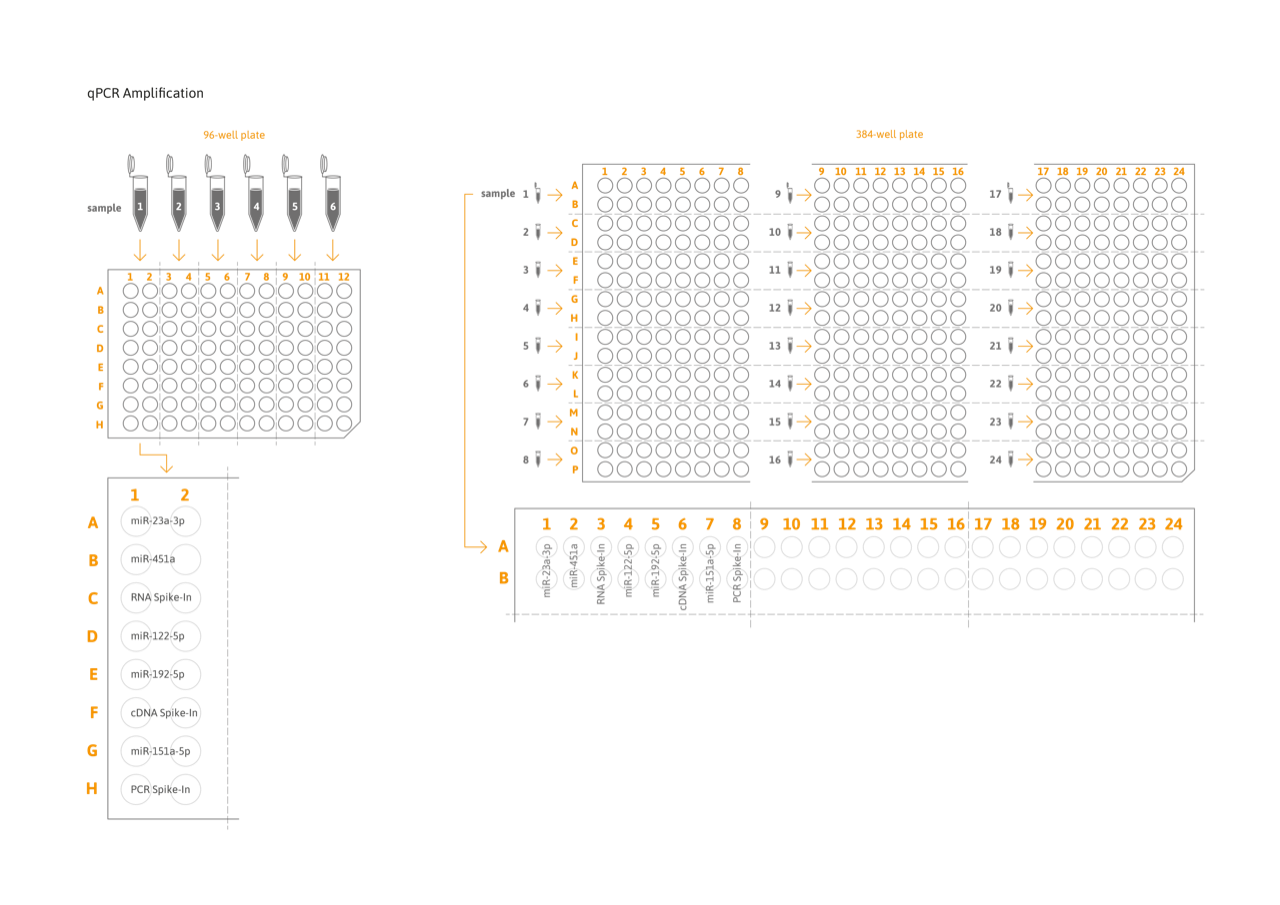
- High throughput: Analysis of up to 6 samples per 96-well or 24 samples per 384-well plate.
- Sample volume:
- 200 µL platelet-poor plasma (lower volumes can be used).
- Samples can be deep-frozen (-70°C or lower) or fresh (centrifuge plasma within 2 hours of blood collection).
- Efficiency by design: duplicate analysis in primer-coated 96- or 384-well plates increases robustness while reducing hands-on time.
- Quality: control assays for sample quality, RNA isolation efficiency and RT-qPCR performance ensure high data quality.
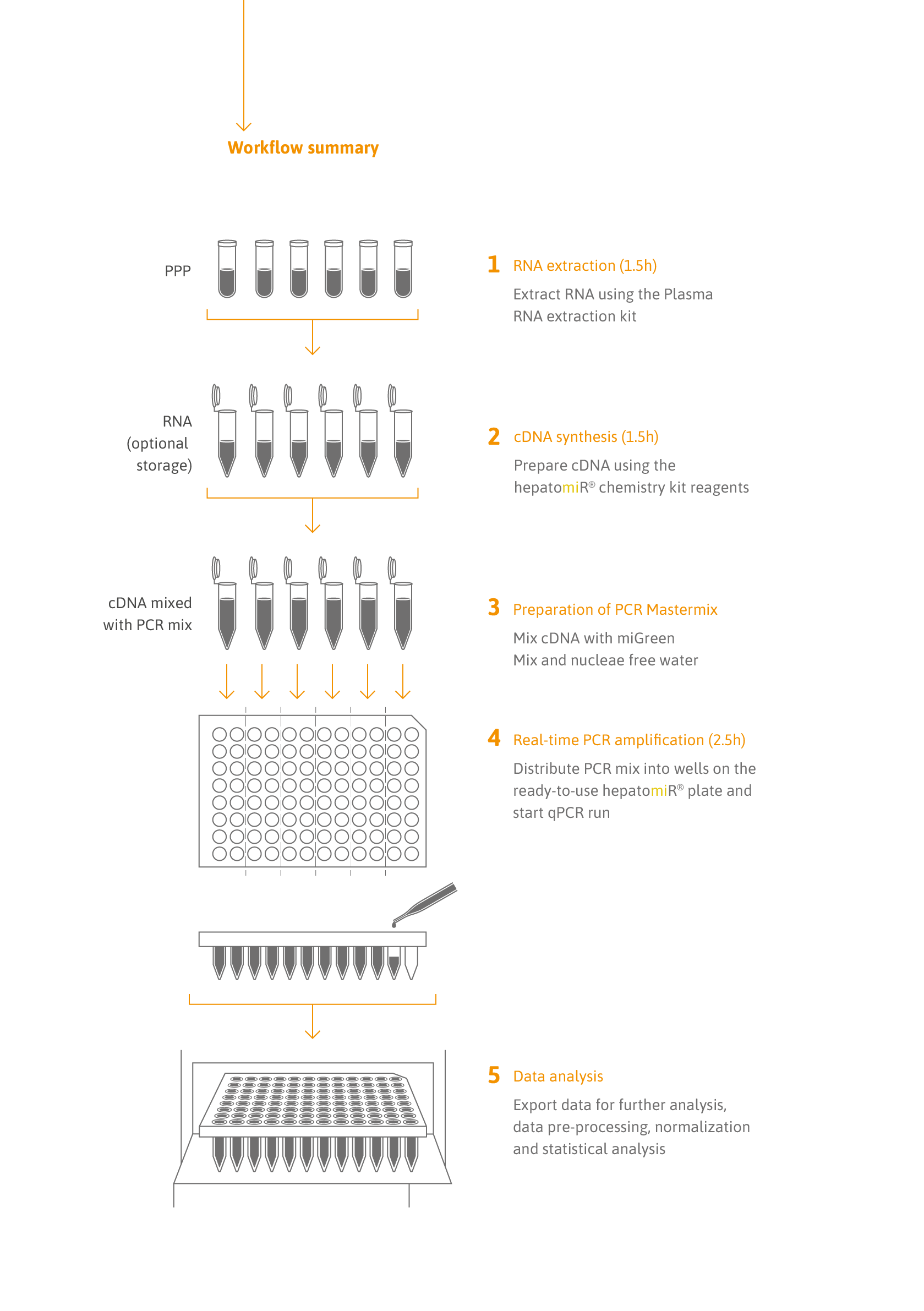
- Fast and simple data analysis: the hepatomiR® software application simplifies data analysis. Going from raw data to a full report takes <5 minutes.
Further information about the components included can be found here
The hepatomiR® software application is intended for:
- Standardized analysis of qPCR data
- Import: upload of raw data files from qPCR instrument and automatic Cq-value calling
- Quality Control: inspect amplification curves, melting temperature, spike-in and hemolysis controls
- Analysis: calculation and interpretation of hepatomiR® risk score
- Export: export of raw Cq-values and hepatomiR® risk score
The hepatomiR® software application and further information will be made available to all registered customers of the hepatomiR® kit.
intended use
The hepatomiR® kit is intended to be used to quantify the levels of three human miRNAs, hsa-miR-122-5p, hsa-miR-192-5p, and hsa-miR-151a-5p in human platelet-poor plasma samples. The hepatomiR® software calculates and returns a score (“P-score”), that can be used as a surrogate of liver function. The hepatomiR® P-score ranges between 0 and 1. A high P-score indicates reduced liver function and higher risk of adverse outcomes.
definition of the patient population
The population used to develop hepatomiR® was men (69%) and women (31%). The age range was 22-90 years with a median of 65 years. Three major tumor types were represented as hepatectomy, specifically hepatocellular (HCC) (20%), cholangiocellular (CCC) (19%), and metastatic colorectal carcinomas (mCRC) (41%). Other tumor types (9%) and benign tumors (5.5%) were represented as well.
other intended use
The hepatomiR® P-score has been found to be potentially useful for preoperative (pre- OP) assessment of liver function in patients undergoing hepatic surgery. Together with other clinical parameters, the hepatomiR® P-score can inform about a patient’s risk of posthepatectomy liver failure (PHLF). Therefore, the test can be applied in patients in need of partial liver resection (for example for the treatment of mCRC, CCC, or HCC cancers) who are eligible for liver resection based on tumor size and distribution, general health status and liver function status according to traditional liver function parameters for the following reasons:
• To identify patients with high risk of PHLF, who will not benefit from operation.
• To identify patients with low risk of PHLF, who can be operated without delay
• To identify patients with intermediate risk of PHLF, who are eligible of preoperative intervention with the aim of improving liver function.
• To monitor of liver function during and after intervention with the aim of improving liver function.
Others planned intended use of the product are: the assessment of liver function in the context of acute or chronic liver diseases.
Key Publications
Starlinger P, Hackl H, Pereyra D, Skalicky S, Geiger E, Finsterbusch M, Tamandl D, Brostjan C, Grünberger T, Hackl M, Assinger A. (2019). Predicting Postoperative Liver Dysfunction Based on Blood Derived MicroRNA Signatures. Hepatology. 2019 Jun;69(6):2636-2651. doi: 10.1002/hep.30572. Epub 2019 Apr 10