news
Eager to lern more about Sepsis-associated acute kidney injury?
We are pleased to share our latest publication, a collaborative effort with TAmiRNA, titled 'Sepsis-Induced Heterogeneous Transcription of Coagulation- and Inflammation-Associated Genes in Renal Microvasculature,' which has been published in Thrombosis Research. Through the integration of TAmiRNA's high-sensitivity gene expression profiling techniques (RT-qPCR and smallRNAseq/RNAseq) and the advanced laser microdissection technology from Dutch company Vivomicx, our study unveils the diverse transcriptional responses of [...]
Exciting news!
We just received the Illumina NextSeq 2000 machine, marking a significant milestone in our journey towards cutting-edge research and innovation. This powerful instrument will drive our NGS services and allow us to deliver results quicker than ever before.
TAmiRNA at RNA Leaders conference in Basel
TAmiRNA CEO/CSO Matthias Hackl will attend the RNA Leaders conference in Basel next week to speak about the recent development of reliable miRNA safety biomarkers and their potential to indicate injury of the liver, kidneys, pancreas, blood vessels, and central nervous system for drug development purposes. Reach out to Matthias directly to meet with him before or after his presentation on Wednesday, March [...]
Webinar – COST Action AtheroNET
Looking forward to sharing our insights from 10+ years of extracellular microRNA (and recently long RNA as well) biomarker discovery and diagnostic test development with the COST Action AtheroNET group. Date: 19.02.2024 Time: 19:30 CET Registration Link: https://lnkd.in/dVDDYKcA Reach out to Matthias directly after his webinar and discuss our latest developments and how TAmiRNA’s miND spike ins and small RNA sequencing service can assist your business! [...]
TAmiRNA ‘2023 In Review’
One last look back at 2023 before we turn our attention to our goals for 2024: last year brought us progress in many areas, from the development of new laboratory and data analyses to the implementation of hepatomiR® in routine clinical diagnostics and the establishment of important partnerships. Our thanks go to the entire (growing) TAmiRNA team, and our customers and partners! We [...]
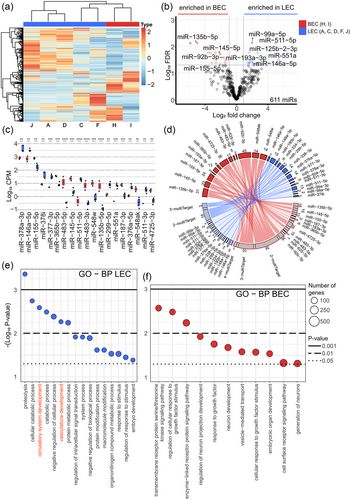
Analysis of extracellular vesicle microRNA profiles reveals distinct blood and lymphatic endothelial cell origins
We want to draw your attention to the latest collaborative publication by TAmiRNA scientist Marianne Pultar: “Analysis of extracellular vesicle microRNA profiles reveals distinct blood and lymphatic endothelial cell origins” published in the Journal of Extracellular Biology. By applying TAmiRNA´s unique small RNA-seq and RNA-seq workflows, this study provides important insights into the secretory behavior of endothelial cell-derived extracellular vesicles (EVs) and their microRNA cargo. In [...]
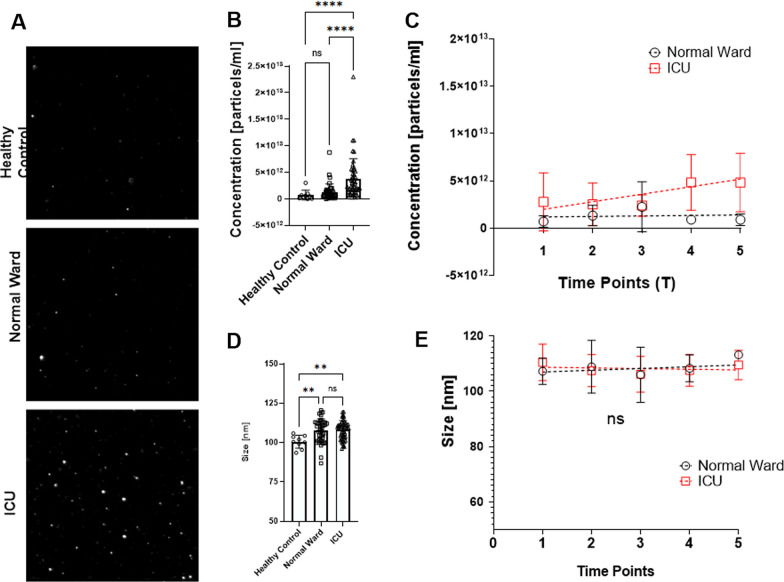
Circulating endothelial extracellular vesicle signatures correspond with ICU requirement
We want to draw your attention to the latest publication in cooperation with TAmiRNA – “Circulating endothelial extracellular vesicle signatures correspond with ICU requirement: an exploratory study in COVID-19 patients” published in Intensive Care Medicine Experimental. Using TAmiRNA’s expertise in the field of EV characterization, we found that endothelial EVs and associated miRNAs could be promising candidates for the diagnosis of patients with COVID-19. Click here [...]
TAmiRNA partners with Techtum Lab
TAmiRNA has entered an exclusive distribution agreement with Techtum Lab for the sales of their products in the Nordics. Techtum Lab is a full service supplier and partner to clinical and research molecular biology laboratories and looks back at >45 years of experience in the Nordic region. TAmiRNA complements Techtum’s NGS portfolio with the miND Spike-Ins, a unique set of controls for small [...]
TAmiRNA meets Japanese Pharma to present microRNA Biomarker Discovery Solutions
Matthias Hackl, CEO/CSO of TAmiRNA, visited Japan between November 11th and 18th to join Summit Pharmaceutical international to introduce TAmiRNA to 12 medium- and large-size pharmaceutical companies. Matthias presented information on microRNA and mRNA biomarker discovery in liquid biopsies using TAmiRNA´s unique miND® pipeline for absolute quantification of microRNAs. In addition, Matthias joined a seminar on “Manufacturing and omic-based characterization of extracellular vesicles [...]
TAmiRNA at MEDICA Düsseldorf
TAmiRNA Senior Scientist Teresa Lara Krammer will attend MEDICA Düsseldorf next week to speak about “thrombomiRs - circulating microRNAs as novel platelet activation markers?”. Reach out to Teresa directly to meet with her before or after her presentation on Tuesday, Nov. 14th at 14:50, Halle1/G37 and discuss our latest developments and how TAmiRNA’s products and services can assist your business! [...]