microRNAs & toxicity
toxicity
MicroRNAs are early indicators and useful tools to detect drug-induced toxicity. Incorporation of miRNA profiling into the drug safety testing process will complement currently used techniques and may substantially enhance drug safety.
While disease-specific biomarkers have found their application in clinical drug development, in particular in oncology, and have been accepted by health authorities in a number of cases, biomarkers for organ toxicity were only very recently endorsed by FDA and European Medicines Agency (EMEA) for use in preclinical toxicity studies (1).
toxicology testing – gold-standard
In the past decades, several well-known clinical pathology parameters associated with toxicity of major organs (liver, kidney, heart) were identified and also routinely applied. On a molecular level, most of these clinical chemistry parameters are enzyme activities or endogenous metabolites which are quantified in serum and/or urine by biochemical methods. These traditional clinical pathology parameters have been recognized to have major drawbacks in terms of specificity and sensitivity and in general often show a limited diagnostic and prognostic performance for early detection of organ injury on toxic insults. Target organ and tissue specificity is the major limitation for most of the traditional serum enzymes used for monitoring of organ integrity and/or function during drug safety assessment (2).
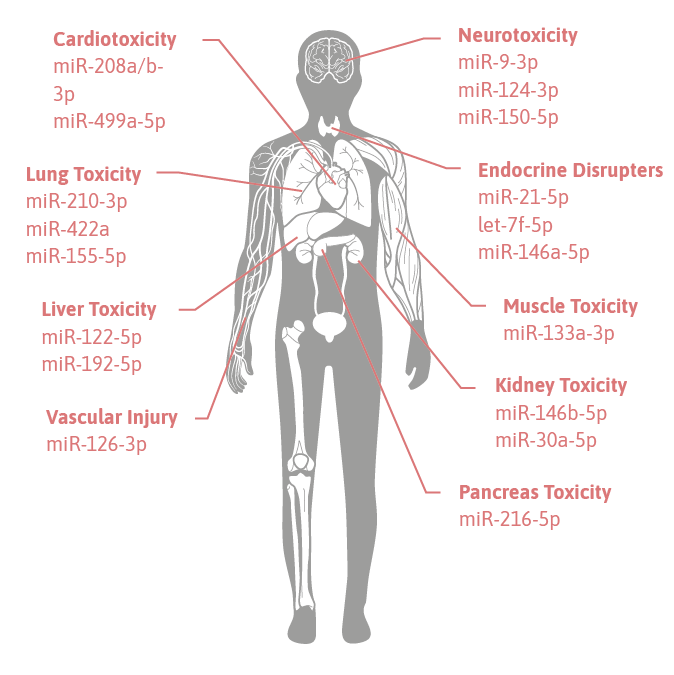
clinical utility for microRNA biomarkers for toxicity
Circulating miRNAs are extremely promising biomarker candidates for toxicological studies, where they are used as biomarkers of drug-, or chemical-induced tissue injury for safety assessment, because
- miRNAs are non-invasive biomarkers for early detection of tissue
- miRNAs regulate gene activity in tissues exposed to toxic substances
- they are novel tools for identifying and monitoring safety risks in drug development
- miRNA are highly-conserved and potentially useful in preclinical animal studies
measurability
Circulating microRNAs can be quantified in serum as well as plasma samples using quantitative PCR. This gold-standard method is reliable and can undergo analytical validation. However, in order to successfully obtain robust results, a well-characterized and standardized protocol must be used. TAmiRNA has developed a robust workflow and offers qPCR kits and services together with software solutions for circulating microRNA analysis.
validated biology
Numerous studies have by now qualified and validated the regulation of miRNAs after toxic injury on multiple tissues and organs. In the following a few examples for so-called “ toxomiRs®” are given:
- miR-122-5p is highly enriched in the liver tissue and exhibit dose- and exposure duration-dependent changes in the plasma that correlate with ALT serum levels and with histopathological changes during liver degeneration (3, 4).
- miR-124-3p is brain-specific and could be used to monitor ischemia-related brain injury (5).
- Plasma miR-208a-3p was robustly increased from the first sampling point through 24 h after treatment with isoproterenol in drug-induced cardiotoxicity (6).
- miR-133a-3p is a specific markers of muscle toxicity. In combination with miR-208a-3p, miR-133a-3p can be used to differentiate cardiac from skeletal muscle toxicity (7).
- miR-30a-5p expression levels can be used to distinguish diabetic nephropathy (DN) from other conditions (8).
- miR-210-3p is commonly upregulated by exposure to three aldehydes, propanal, butanal and pentanal in lung toxicity (9).
- miR-216-5p is pancreas specific, leak into the circulation from the injured pancreatic cells and might serve as a good biomarker for pancreatic injury (10).
- miR-126-3p is specifically expressed in the endothelium and serve as biomarker in vascular injury.
decision support
The toxomiR® panel allows to analyze 19 distinct microRNAs with specific biological functions. Therefore, the toxomiR® panel reports a novel measure toxicity.
For more information have a look on our TechNote TN-06 “Circulating microRNAs as biomarkers of toxicity”








