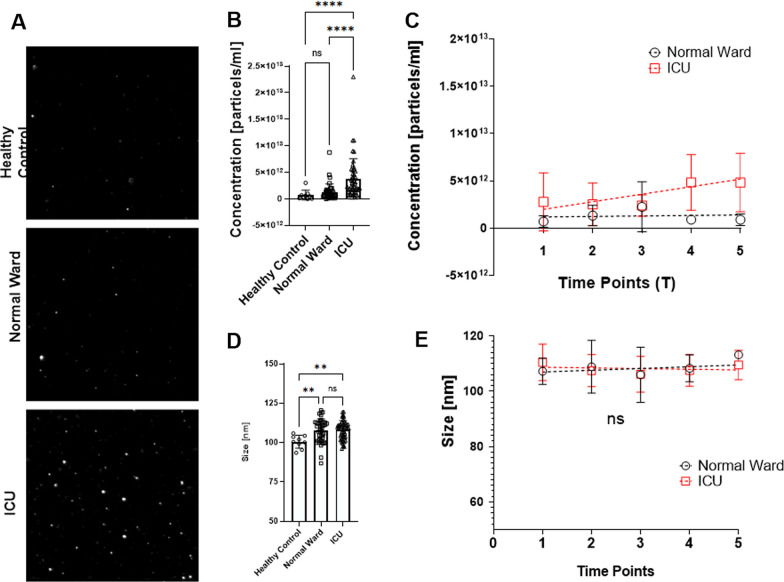
Circulating endothelial extracellular vesicle signatures correspond with ICU requirement
We want to draw your attention to the latest publication in cooperation with TAmiRNA – “Circulating endothelial extracellular vesicle signatures correspond with ICU requirement: an exploratory study in COVID-19 patients” published in Intensive Care Medicine Experimental. Using TAmiRNA’s expertise in the field of EV characterization, we found that endothelial EVs and associated miRNAs could be promising candidates for the diagnosis of patients with COVID-19. Click here to learn more about this service click here to read the paper