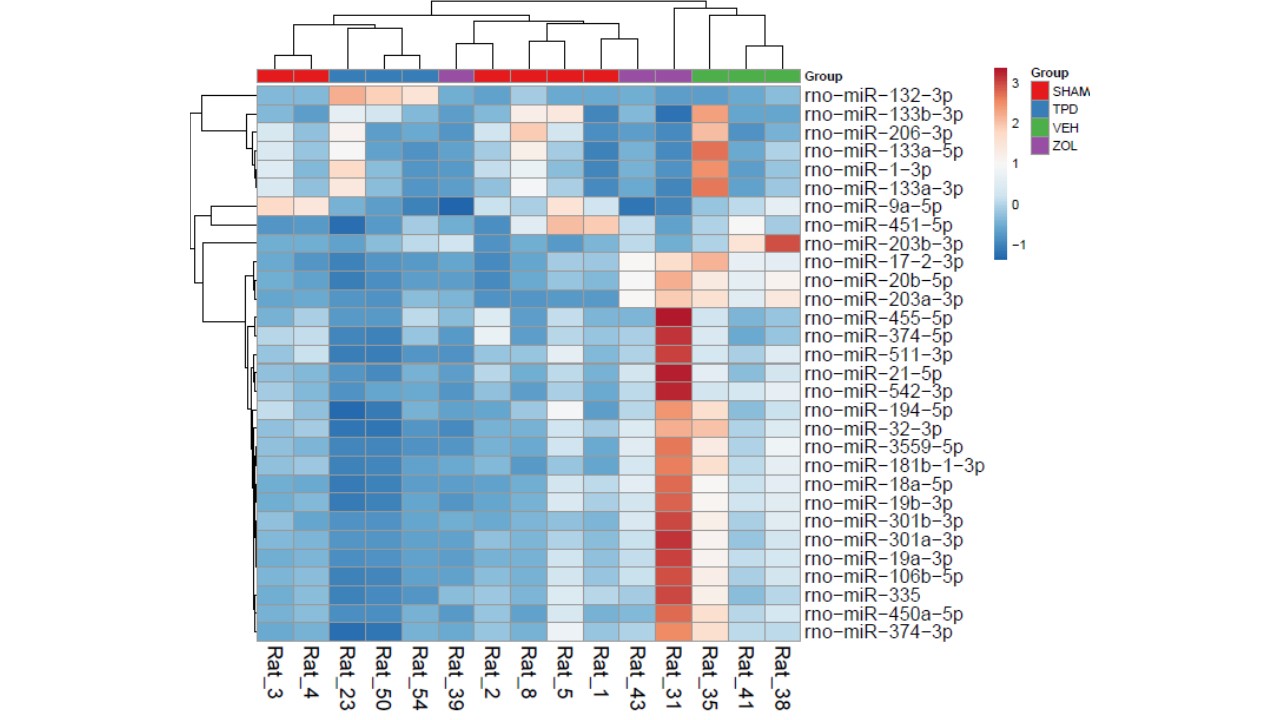
MicroRNA levels in bone and blood change during bisphosphonate and teriparatide therapy in an animal model of postmenopausal osteoporosis.
We want to draw your attention to the latest publication of the Ludwig Boltzmann Institute for Experimental and Clinical Traumatology in AUVA Research Center, in cooperation with TAmiRNA GmbH, in Bone. "MicroRNA levels in bone and blood change during bisphosphonate and teriparatide therapy in an animal model of postmenopausal osteoporosis." Kocijan R, Weigl M, Skalicky S, et al. Bone 2019 Volume 131, February 2020, 115104. https://doi.org/10.1016/j.bone.2019.115104 Take the chance and download the paper